Deafness in Cavalier King Charles Spaniels
Can be Congenital or Progressive
-
 What
It Is
What
It Is - -- Congenital Deafness
- -- PSOM
- -- Progressive Deafness
- -- Other Causes
- Diagnosis
- -- PSOM as a differential diagnosis
- Treatment
- Breeders' Responsibilities
- What You Can Do
- Research News
- Related Links
- Veterinary Resources
Many cavalier King Charles spaniels tend to lose their hearing relatively early in their lives, with some becoming completely deaf by their mid-years, ages 4 to 7 years.
What It Is
There are several possible causes of deafness in dogs and specifically in cavalier King Charles spaniels. We list two documented hereditary causes here -- congenital deafness and primary secretory otitis media (PSOM) -- as well as one other possible hereditary cause -- progressive hearing loss -- and a couple of non-hereditary ones -- anesthesia and noise-induced hearing loss (NIHL).
• Congenital deafness
Cavalier King Charles spaniels are predisposed to a
form of congenital deafness, although it is not as common in cavaliers
as it is in many other breeds. Congenital deafness is present at birth,
due to a lack of formation or
 early degeneration of receptors in the
inner ear. Cavaliers appear to also carry the piebald gene, so dogs in
the breed are potentially subject to pigment-associated congenital
sensorineural deafness, which should be evident in the puppy age span.
early degeneration of receptors in the
inner ear. Cavaliers appear to also carry the piebald gene, so dogs in
the breed are potentially subject to pigment-associated congenital
sensorineural deafness, which should be evident in the puppy age span.
Pigment-associated deafness is congenital and is the most common form of deafness in most breeds of dogs, although not in the CKCS. It is a cochleo-saccular deafness in the cavalier that is associated with the genes which cause white pigmentation (piebald). These genes suppress melanocyte function. Melanocyte is a cell in the bottom layer of the skin which produces the pigment melanin. Melanin is primarily responsible for skin and coat color. When melanocyte function is suppressed, the result is that white becomes the default color. Melanocyte also is located in the inner ear, bones, heart, and elsewhere in the dog’s body. All white cavaliers have been produced from purebred CKCS stock, and in all identified cases, they have suffered congenital deafness.
Another form of congenital deafness in the CKCS -- abiotrophic sensorineural deafness -- is discussed separaely below.
• PSOM
Another ear disorder becoming more common in cavaliers is Primary Secretory Otitis Media (PSOM), also called "glue ear", which can produce a conductive hearing loss, due to the mucus plug which PSOM produced in the dog's inner ear. The mucus accumulation in the middle ear results in loss of conduction of sound waves which reduces the ability of the middle ear bones to transfer the sound wave energy to the inner ear hearing apparatus, the cochlea. As such, the first signs are often related to changes in hearing threshold, with many dogs responding to louder noises only. Over time, complete hearing loss may occur. PSOM is discussed at length on its own webpage.
• Progressive deafness
 In addition,
studies in the 1990s by
Dr. Michael Podell
(right)
had suggested that cavaliers may develop a progressive hereditary hearing
loss, which usually begins during puppyhood and worsens, or progresses,
until the dog is completely deaf, usually between the ages of three and
five years. The progressive nature of this form of deafness in CKCSs was
believed to be due to degeneration of the hearing nerve, rather than the
lack of formation or early degeneration of the inner ear receptors. Dr.
Podell also had referred this
disorder, in a brief abstract, as abiotrophic sensorineural deafness and has described it as
an inherited congenital sensorineural deafness which affects hair cells
of the spiral organ. It does not appear that he published any peer-reviewed articles
on this topic. Drs.
Alexander de Lahunta and
Eric N. Glass
also reported on abiotrophic
sensorineural deafness in the breed, stating:
In addition,
studies in the 1990s by
Dr. Michael Podell
(right)
had suggested that cavaliers may develop a progressive hereditary hearing
loss, which usually begins during puppyhood and worsens, or progresses,
until the dog is completely deaf, usually between the ages of three and
five years. The progressive nature of this form of deafness in CKCSs was
believed to be due to degeneration of the hearing nerve, rather than the
lack of formation or early degeneration of the inner ear receptors. Dr.
Podell also had referred this
disorder, in a brief abstract, as abiotrophic sensorineural deafness and has described it as
an inherited congenital sensorineural deafness which affects hair cells
of the spiral organ. It does not appear that he published any peer-reviewed articles
on this topic. Drs.
Alexander de Lahunta and
Eric N. Glass
also reported on abiotrophic
sensorineural deafness in the breed, stating:
"Congenital abiotrophic sensorineural deafness directly affects the hair cells in the spiral organ. ... The lesion is usually bilateral and occasionally also affects the hair cells of the vestibular system receptors. The inheritance usually involves an autosomal recessive gene. Although most of these abiotrophies cause deafness at a few weeks of age, occasionally the onset of deafness occurs later in life. In the cavalier King Charles spaniel, deafness may not be apparent until 3 or 4 years of age."
However, Dr. Podell's analysis pre-dated the 2003 study led by Dr. Wiwian Stern Bertholtz which diagnosed 61 cases of PSOM in 43 CKCSs. In the 1990s, most BAER testers did not have or use bone-stimulation transducers that permitted distinguishing between sensorineural and conductive deafness, and so diagnoses were based on flat-line air-conducted stimuli BAER examinations which were interpreted to be sensorineural. Since then, the consensus opinion is that cavaliers do not suffer from an adult-onset abiotrophic sensorineural deafness, but rather from PSOM.
EDITOR'S NOTE: Notwithstanding the consensus opinion that PSOM is the more likely cause of this progressive deafness, rather than the independent cause (abiotrophic sensorineural deafness affecting hair cells of the spiral organ), we have received numerous reports of CKCSs gradually losing their hearing over a period of years, beginging in early adulthood and ending by the 4th or 5th year, without there being any detected evidence of PSOM. We believe that progressive deafness in many cavaliers is a separate disorder from PSOM.
• Other causes
• ANESTHESIA: In a July 2010 article, Dr. George Strain reported permanent hearing loss to 62 dogs and cats -- none being cavaliers -- following anesthesia for dental and ear cleaning procedures.
• LOUD NOISES: Noise-induced hearing loss (NIHL) is the result of loud noises, such as gunfire and explosions. The NIHL effect is cumulative and irreversible. It shows up in military and police dogs and also in dogs kept in shelters or kennels where noise levels are high. Loud sounds can damage the ear drums (tympanic membrane) or the bones of the inner ear or the hair cells that line the inner ear and transmit the noise to the brain. Loud noises can also induce a very loud ringing in the ears (tinnitus) which may subside over time. Sound levels above 120-140 dB can cause hearing loss.
• PRESBYCUSIS: Age-related hearing loss, called presbycusis by veterinarians, is the most common form among all breeds of dogs. Typicaly, this loss of hearing begins around 8–10 years of age. It appears to be caused by changes in the organ of Corti, stria vascularis, and basilar membrane. It is most pronounced in the middle to high-frequency ranges.
RETURN TO TOP
Diagnosis
Diagnosis may be conducted using a variety of modalities. The
examining veterinarian usually begins with a
 lighted
otoscope (left),
which he would insert manually in the ear canal to observe as much as is
visible, down to the dog's tympanic membrane (eardrum).
lighted
otoscope (left),
which he would insert manually in the ear canal to observe as much as is
visible, down to the dog's tympanic membrane (eardrum).
The conventional manner of testing a dog's hearing is the BAER (for Brainstem Auditory Evoked Response) test. The BAER test is non-invasive and provides an objective assessment of auditory function in canines and other animals. The BAER test objectively examines a dog's hearing by bypassing the need to rely subjectively on the patient's response.
(Photo at right is of a cavalier having a BAER test. Courtesy of Jen Vanasse B.A.E.R. Testing.)
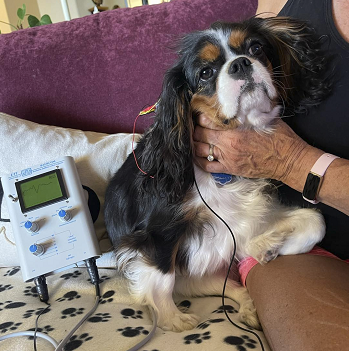 BAER measures the timing of electrical waves from the
brain stem in response to a click, as a sound stimulus in the ear
travels to the brain.
Within milliseconds of each click being made in a hearing dog's ear, a
series of standard electrical waves appear on the BAER instrument's
screen. There are 7 waves, of which waves I, II, III, and V are
significant in determining any abnormalities.
BAER measures the timing of electrical waves from the
brain stem in response to a click, as a sound stimulus in the ear
travels to the brain.
Within milliseconds of each click being made in a hearing dog's ear, a
series of standard electrical waves appear on the BAER instrument's
screen. There are 7 waves, of which waves I, II, III, and V are
significant in determining any abnormalities.
The first wave (wave I) comes from a nerve which transmits sound information to the brain. The audiologist evaluates the latency (distance or time between peaks of the waves) and amplitude (height of the waves). Then waves II, III, and V come from the areas of the brain stem which generate the hearing signal to the front of the brain and then to the cerebrum where the signal is interpreted as a sound. If the dog cannot hear the clicks, the waves will not appear on the screen. There are two types of BAER tests, air-conducted and bone-conducted. (Below is a BAER scan of a cavalier with hearing loss.)
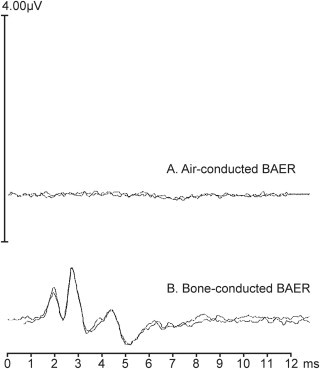 However,
conventional BAER testing usually does not adequately identify cavaliers
with progressive hearing deficiencies, because most BAER sites test only
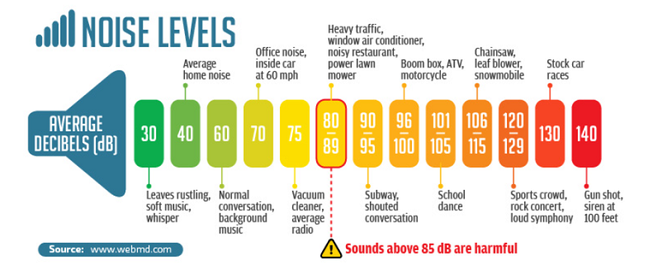
from a peak equivalent Sound Pressure Level (pe SPL) intensity threshold of 70 to 90
decibels (dB), which is about the level of the sound of a vacuum cleaner next
to the ear. (See Noise Levels Chart, below) The human voice normally is at the 60 dB intensity level, so
even a cavalier which passes the conventional BAER test could be deaf to
human voices and levels of normal daily sounds.
However,
conventional BAER testing usually does not adequately identify cavaliers
with progressive hearing deficiencies, because most BAER sites test only
from a peak equivalent Sound Pressure Level (pe SPL) intensity threshold of 70 to 90
decibels (dB), which is about the level of the sound of a vacuum cleaner next
to the ear. (See Noise Levels Chart, below) The human voice normally is at the 60 dB intensity level, so
even a cavalier which passes the conventional BAER test could be deaf to
human voices and levels of normal daily sounds.
Ideally, BAER tests of cavaliers should start at a high intensity, such as 116 dB, and decrease by about 10 dB at a time to as low a decibel intensity threshold as possible – no higher than 30 dB.
In an October 2022 abstract, Ohio State Univ. veterinary hearing specialists evaluated the hearing of 20 cavaliers, all without apparent hearing abnormalities, to determine the BAER testing parameters of CKCSs, based upon their grades of Chiari-like malformation (CM) -- CM0 (no Chiari malformation), and CM1 (cerebellum indented but not rounded), and CM2 (cerebellum impacted into, or herniated through, the opening at the rear of the skull, the foramen magnum). (For more information on the grading of CM in cavaliers, see our SM Protocol webpage.) They assessed the cavaliers using BAER, and CT scans (to assess the middle ear), and MRI scans (to assess presence and grade of CM). They report finding that of the 20 CKCSs, all had CM, with 9 (45%) having the milder form of CM1 and 11 (55%) having the more severe form of CM2. They also made these important findings regarding future BAER testing of cavaliers:
• Median threshold for CKCS with CM1 was 39 decibels (dB) and CM2 was 46 dB.
• Absolute latencies (distance or time between peaks of the waves) for CKCS with CM2 were consistently longer than those for CKCS with CM1 with exception of Wave II, V at 33 dB.
• Significant differences were found for wave V at 102 dB, wave II at 74 dB.
• Interpeak latency comparisons were inconsistent between CM1 and CM2.
They concluded that: "Normative BAER data for CKCS with CM1 and CM2 were established. Results suggest CM impacts BAER latency results, but influence is not always statistically significant or predictable." (See, also, this group's May 2023 article on the same research.)
Click here for a list of BAER test sites. Also, check our health clinics webpage for upcoming clinics offering BAER tests. Look for the symbol U. The University of Cincinnati in Ohio sponsors a canine hearing laboratory, FETCH~LAB, which is dedicated to canine hearing management and related studies, including animal audiology,animal vocal mechanisms, noise impacts to animal hearing, and bioacoustics. See FETCH~LAB's website.
CKCSs may be tested as early as eight weeks of age for congenital deafness, once the puppies’ ear canals are completely open. However, the condition of progressive hearing loss – due to degeneration of the hearing nerve – has a later onset and cannot be detected in young puppies.
• PSOM as a differential diagnosis
 Primary secretory otitis media (PSOM) is
frequently diagnosed in cavalier King Charles spaniels, and the mucus
plug, which PSOM creates in the inner ear, can cause a conductive
hearing loss. BAER testing is able to diagnose hearing losses, but it
can be inconclusive as to whether the dog has a conductive hearing loss
or a sensorineural hearing loss or a combination of the two.
Therefore, if a cavalier is suspected to have impaired hearing, it is necessary to determine if the hearing loss
is conductive (with a likely cause of PSOM) or sensorineural (due to
abiotrophic sensorineural deafness), or both.
Primary secretory otitis media (PSOM) is
frequently diagnosed in cavalier King Charles spaniels, and the mucus
plug, which PSOM creates in the inner ear, can cause a conductive
hearing loss. BAER testing is able to diagnose hearing losses, but it
can be inconclusive as to whether the dog has a conductive hearing loss
or a sensorineural hearing loss or a combination of the two.
Therefore, if a cavalier is suspected to have impaired hearing, it is necessary to determine if the hearing loss
is conductive (with a likely cause of PSOM) or sensorineural (due to
abiotrophic sensorineural deafness), or both.
To do this, hearing specialists typically will evaluate for PSOM using the standard procedures discussed on its webpage. Briefly, this consists of first performing an otic examination by manually using an otoscope. If that exam does not show that the top part of the ear drum is bulging (indicating PSOM), then the veterinarian will do a computed tomography (CT) scan or a magnetic resonance imaging (MRI) scan under anesthesia, still trying to diagnose or eliminate PSOM.
If the cavalier has PSOM, the specialist then normally will perform the two BAER tests (air-conducted and bone-conducted) after the scan, to see if there is hearing loss and try to assess whether it is conductive or sensorineural. If the cavalier does not have PSOM, the likelihood of a conductive hearing loss will be discounted, and the specialist will perform the air-conducted BAER test. If that test diagnoses that the dog's hearing is impaired, the hearing loss would be classified as sensorineural.
RETURN TO TOP
Treatment
Most causes of loss of hearing are not reversible or otherwise treatable. An exception is PSOM, which as noted above, is discussed at length on its own webpage.
A February 2017 article suggests that the loss of hearing due to the destruction of cochlear hair cells, which do not naturally regenerate, may be treated by the introduction of stem cells which have caused the generation of hair cells in the cochlea of mice.
RETURN TO TOP
Breeders' Responsibilities
No cavalier suffering from an hereditary hearing deficiency should be bred. BAER testing should be conducted at no younger than age 2.5 years, and at the low decibel intensity threshold of 30 dB, to assure that the cavaliers do not suffer from the hereditary disease of deafness which they would pass along to their litters.
RETURN TO TOP
What You Can Do
There
is a possibility that any cavalier will become deaf or partially
deaf. You can prepare for this possibility by
 learning how to
communicate with your dog by alternative ways, especially hand
signals and purposeful body motions. If a dog knows how to otherwise
understand what you expect of him, before he becomes deaf, then
communicating with him after he loses his hearing will be much
easier.
learning how to
communicate with your dog by alternative ways, especially hand
signals and purposeful body motions. If a dog knows how to otherwise
understand what you expect of him, before he becomes deaf, then
communicating with him after he loses his hearing will be much
easier.
There are books and websites which offer helpful advice in communicating with deaf dogs. An excellent such book is Living With a Deaf Dog: A Book of Advice, Facts and Experiences About Canine Deafness by Susan C. Becker. An excellent website is DeafDogs.org
 In the meantime, keep your dogs' ears clean. Here is a
YouTube video that shows how it can be done. We recommend ear
cleaning products like
Vet's + Best Ear Relief Wash + Dry and using them every week to
ten days.
In the meantime, keep your dogs' ears clean. Here is a
YouTube video that shows how it can be done. We recommend ear
cleaning products like
Vet's + Best Ear Relief Wash + Dry and using them every week to
ten days.
If you know that your cavalier is going to be in proximity of loud noises, such as gunfire, explosives, and high noise level living conditions, a preventative option would be sound-resistant ear muffs. One such product, Mutt Muffs, has been proven to be effective and comfortable for many dogs.
RETURN TO TOP
Research News
October 2022:
Cavaliers' BAER hearing test results are affected by the dogs'
grades of Chiari-like malformation.
 In an
October 2022 abstract, Ohio State Univ. veterinary hearing
specialists (Lynette K. Cole [right], Susan Wagner, Sarah
Moore, Ronaldo da Costa, Eric Hostnik, Laura Selmic) evaluated the
hearing of 20 cavalier King Charles spaniels, all without apparent
hearing abnormalities, to determine the brainstem auditory evoked
response (BAER) testing parameters of CKCSs, based upon their grades of
Chiari-like malformation (CM) -- CM0 (no Chiari malformation), and CM1
(cerebellum indented but not rounded), and CM2 (cerebellum impacted
into, or herniated through, the opening at the rear of the skull, the
foramen magnum). (For more information on the grading of CM in
cavaliers, see our SM Protocol webpage.) They assessed the cavaliers
using BAER, and CT scans (to assess the middle ear), and MRI scans (to
assess presence and grade of CM). They report finding that of the 20
CKCSs, all had CM, with 9 (45%) having the milder form of CM1 and 11
(55%) having the more severe form of CM2. They also made these important
findings regarding future BAER testing of cavaliers:
In an
October 2022 abstract, Ohio State Univ. veterinary hearing
specialists (Lynette K. Cole [right], Susan Wagner, Sarah
Moore, Ronaldo da Costa, Eric Hostnik, Laura Selmic) evaluated the
hearing of 20 cavalier King Charles spaniels, all without apparent
hearing abnormalities, to determine the brainstem auditory evoked
response (BAER) testing parameters of CKCSs, based upon their grades of
Chiari-like malformation (CM) -- CM0 (no Chiari malformation), and CM1
(cerebellum indented but not rounded), and CM2 (cerebellum impacted
into, or herniated through, the opening at the rear of the skull, the
foramen magnum). (For more information on the grading of CM in
cavaliers, see our SM Protocol webpage.) They assessed the cavaliers
using BAER, and CT scans (to assess the middle ear), and MRI scans (to
assess presence and grade of CM). They report finding that of the 20
CKCSs, all had CM, with 9 (45%) having the milder form of CM1 and 11
(55%) having the more severe form of CM2. They also made these important
findings regarding future BAER testing of cavaliers:
• Median threshold for CKCS with CM1 was 39 decibels (dB) and CM2 was 46 dB.
• Absolute latencies (distance or time between peaks of the waves) for CKCS with CM2 were consistently longer than those for CKCS with CM1 with exception of Wave II, V at 33 dB.
• Significant differences were found for wave V at 102 dB, wave II at 74 dB.
• Interpeak latency comparisons were inconsistent between CM1 and CM2.
They concluded that: "Normative BAER data for CKCS with CM1 and CM2 were established. Results suggest CM impacts BAER latency results, but influence is not always statistically significant or predictable." (See, also, this group's May 2023 article on the same research.)
September 2016:
Univ. of Cincinnati's FETCH~LAB offers hearing tests for dogs.
 The
University of Cincinnati in Ohio sponsors a canine hearing laboratory,
FETCH~LAB, which is dedicated to canine hearing management and
related studies, including animal audiology,animal vocal mechanisms,
noise impacts to animal hearing, and bioacoustics. It currently is
offering full service hearing tests for dogs, consisting initially of
brainstem auditory response (BAER), which provides an objective and
quantitative measure of the electrical potential produced by the brain
in response to sound by frequency, and otoacustic emissions (OAE)
testing to measure the ear’s production of small echoes in response to
sounds introduced into the ear canal. If necessary, the dogs also may
undergo diagnostic imaging studies to pinpoint the disorder’s cause and
suggest management options. For more information, check out the
laboratory's website.
The
University of Cincinnati in Ohio sponsors a canine hearing laboratory,
FETCH~LAB, which is dedicated to canine hearing management and
related studies, including animal audiology,animal vocal mechanisms,
noise impacts to animal hearing, and bioacoustics. It currently is
offering full service hearing tests for dogs, consisting initially of
brainstem auditory response (BAER), which provides an objective and
quantitative measure of the electrical potential produced by the brain
in response to sound by frequency, and otoacustic emissions (OAE)
testing to measure the ear’s production of small echoes in response to
sounds introduced into the ear canal. If necessary, the dogs also may
undergo diagnostic imaging studies to pinpoint the disorder’s cause and
suggest management options. For more information, check out the
laboratory's website.
February 2016:
Ohio State University's Lynette Cole is conducting study of cavaliers with
no hearing loss.
 Dr. Lynette Cole of The Ohio State
University's Veterinary Medical Center is conducting a study to obtain
data from normal hearing young cavalier King Charles spaniels (CKCS)
with no history of hearing loss. The two-day test using Brainstem
Auditory Evoked Response (BAER) equipment, which measures the timing of
electrical waves from the brain stem in response to a click, as a sound
stimulus, in the ear. A computed tomography (CT) scan and a magnetic
resonance imaging (MRI) scan also will be performed. The study pays for all testing expenses. Dr. Cole
explains:
Dr. Lynette Cole of The Ohio State
University's Veterinary Medical Center is conducting a study to obtain
data from normal hearing young cavalier King Charles spaniels (CKCS)
with no history of hearing loss. The two-day test using Brainstem
Auditory Evoked Response (BAER) equipment, which measures the timing of
electrical waves from the brain stem in response to a click, as a sound
stimulus, in the ear. A computed tomography (CT) scan and a magnetic
resonance imaging (MRI) scan also will be performed. The study pays for all testing expenses. Dr. Cole
explains:
"Hearing disorders are a common condition recognized in many breeds of dogs. In the dog breed Cavalier King Charles Spaniel (CKCS), hearing disorders may be due to conductive hearing loss, which may occur with primary secretory otitis media (PSOM), or do to sensorineural hearing loss, which may occur when there is damage or an abnormality of the sensory cells in the cochlea or the auditory nerve. Evaluation of a dog’s hearing ability is done using the brain-stem auditory evoked response (BAER) test. However, in order to identify an abnormality on the BAER test, the results from an individual dog must be compared to normal BAER values."
If interested in possibly enrolling a CKCS in the study, contact Dr. Cole at 614-247-8706 or cole.143@osu.edu. A pedigree is required for entry into the study, but will be kept confidential. Details of the study will be given individually on the phone or via email. See this explanatory webpage.
July 2014: UK's Dr. Jacques Penderis finds MRI noise results in some hearing loss in dogs. In a study of 36 dogs, a Univerisity of Glasgow team lead by Dr. Jacques Penderis found that exposure to noise during MRI scans results in significant reductions in cochlear function at five of fourteen frequencies. It is not known whether the hearing loss is reversible or permanent. See more details in the November 2013 entry below.
November 2013:
Univ. of Glasgow thesis reports MRI noise causes hearing loss and reduced cochlear function in dogs. In a
2013 Master of Science (Research) thesis at the University of Glasgow,
Rebecca Elisabeth Venn (right) reports that all of 36 dogs (including four cavalier King
Charles spaniels) which underwent MRI scans, experienced reduced cochlear
function and more than half of the 36 dogs had hearing loss using Distortion
Product Otoacoustic Emissions (DPOAE) testing. Ms. Venn noted that the post-MRI
testing was not repeated weeks after the MRIs, so it is not known if the hearing
loss was temporary or permanent. She also noted that evidence from human MRI
noise exposure suggests that the hearing loss is temporary. She recommends that
"all dogs having MRI studies performed should have ear protection as a standard
precautionary measure." See also the
July 2014 entry above.
In a
2013 Master of Science (Research) thesis at the University of Glasgow,
Rebecca Elisabeth Venn (right) reports that all of 36 dogs (including four cavalier King
Charles spaniels) which underwent MRI scans, experienced reduced cochlear
function and more than half of the 36 dogs had hearing loss using Distortion
Product Otoacoustic Emissions (DPOAE) testing. Ms. Venn noted that the post-MRI
testing was not repeated weeks after the MRIs, so it is not known if the hearing
loss was temporary or permanent. She also noted that evidence from human MRI
noise exposure suggests that the hearing loss is temporary. She recommends that
"all dogs having MRI studies performed should have ear protection as a standard
precautionary measure." See also the
July 2014 entry above.
September 2012:
UK Dr. Gert ter Haar heads RVC's new ear, nose, and throat clinic.
 UK's Royal Veterinary College has commenced an ear, nose, and throat
referral clinic at the Queen Mother Hospital for Animals in London. Dr. Gert ter Haar
(right) heads the unit, which offers services
including advanced diagnostics and treatment for PSOM in cavaliers, as
well as CT and BAER diagnostics for deafness (plus hearing aid implants
for selected patients), and diagnostics and treatment of cavaliers with
brachycephalic airway obstruction syndrome (BAOS). Telephone 01707 666
365 for more information.
UK's Royal Veterinary College has commenced an ear, nose, and throat
referral clinic at the Queen Mother Hospital for Animals in London. Dr. Gert ter Haar
(right) heads the unit, which offers services
including advanced diagnostics and treatment for PSOM in cavaliers, as
well as CT and BAER diagnostics for deafness (plus hearing aid implants
for selected patients), and diagnostics and treatment of cavaliers with
brachycephalic airway obstruction syndrome (BAOS). Telephone 01707 666
365 for more information.
 May 2012:
OSU’s Dr. Cole needs
normal hearing CKCSs for BAER tests, CT scans, and MRI scans.
Dr. Lynette Cole (right) of Ohio State University’s
Veterinary Dermatology and Otology Service needs cavaliers between the
ages of 1 to 2 years old with no history of hearing loss for a two-day
evaluation by BAER hearing tests, computed tomography (CT) scans, and
MRI scans. The study pays for all testing. She writes:
May 2012:
OSU’s Dr. Cole needs
normal hearing CKCSs for BAER tests, CT scans, and MRI scans.
Dr. Lynette Cole (right) of Ohio State University’s
Veterinary Dermatology and Otology Service needs cavaliers between the
ages of 1 to 2 years old with no history of hearing loss for a two-day
evaluation by BAER hearing tests, computed tomography (CT) scans, and
MRI scans. The study pays for all testing. She writes:
“In the dog breed Cavalier King Charles Spaniel (CKCS), hearing disorders may be due to conductive hearing loss, which may occur with primary secretory otitis media (PSOM), or due to sensorineural hearing loss, which may occur when there is damage or an abnormality of the sensory cells in the cochlea or the auditory nerve.”
If you are interested in possibly enrolling your cavalier in the study, contact Dr. Cole at telephone number 614-292-3551 or email cole.143@osu.edu. Click here for the OSU webpage. A pedigree is required for entry into the study, but will be kept confidential, as will all test results. Details of the study will be given individually on the phone or via email.
April 2011: Researchers seek DNA swabs to study hearing loss in cavaliers. The Canine Genetics Group at the Van Andel Research Institute and the Translational Genomics Research Institute is studying DNA patterns that contribute to hearing loss in the CKCS. Thus far, the group has identified such DNA patterns for four other breeds. If you have a cavalier suspected of hearing loss, a saliva sample is all the group needs for the study. If you have an older cavalier with good hearing, the study needs DNA from those dogs, too, for "control" purposes. To obtain a free DNA kit, contact the Program for Canine Health and Performance at dogdna@tgen.org Include your mailing address and tell them the number of kits you need for both affected cavaliers and healthy cavaliers. Blood samples from affected CKCSs, drawn by veterinarians, also are needed. Contact Elissa Boguslawski at elissa.boguslawski@vai.org for blood sample submission instructions.
RETURN TO TOP
Related Links
RETURN TO TOP
Veterinary Resources
Investigation
of hearing impairment in Cavalier King Charles spaniels,
using auditory brainstem response audiometry.
Munro, K.J., Cox, C.L. J. Small Animal Prac. January 1997; doi:
10.1111/j.1748-5827.1997.tb02976.x. Quote: Auditory brainstem response
[ABR] audiometry was used to
investigate nine Cavalier King Charles spaniels
 [between
the age of 14 and 93 months] with a history of hearing impairment.
Successful recordings were made in all cases. ... The ABR threshold
in a normal hearing CKCS appears to be around 20 dB
nHL. ... In eight of the dogs, the hearing impairment was between 40
and 85 decibels re normal hearing level. ... In the control group,
thresholds for the better ear were generally between 10 and 20 dB
nHL. In the affected group, where the owners had expressed concern
about their dogs hearing, the ABR threshold in the better ear varied
from 40 to 85 dB nHL except in one dog (case 4) which had a better
ear threshold of 30 dB nHL. ... In addition to confirming the degree
of impairment in each ear, information was obtained concerning the
site of the lesion. The auditory brainstem technique may have an
important role to play in assessing treatment outcome. Other
applications include screening animals used in breeding programmes
as well as working dogs requiring good binaural hearing. ...
The information concerning normal hearing sensitivity has already
proved useful with additional dogs. Four CKCSs whose owners were
concerned about their dogs’ hearing have been tested subsequent to
this study. These dogs varied in age from four to eight years.
Hearing levels in the better ear were 55 dB nHL in three dogs and 80
dB nHL in the fourth dog. (Fig. 3. A dog set up for
auditory brainstem response hearing.)
[between
the age of 14 and 93 months] with a history of hearing impairment.
Successful recordings were made in all cases. ... The ABR threshold
in a normal hearing CKCS appears to be around 20 dB
nHL. ... In eight of the dogs, the hearing impairment was between 40
and 85 decibels re normal hearing level. ... In the control group,
thresholds for the better ear were generally between 10 and 20 dB
nHL. In the affected group, where the owners had expressed concern
about their dogs hearing, the ABR threshold in the better ear varied
from 40 to 85 dB nHL except in one dog (case 4) which had a better
ear threshold of 30 dB nHL. ... In addition to confirming the degree
of impairment in each ear, information was obtained concerning the
site of the lesion. The auditory brainstem technique may have an
important role to play in assessing treatment outcome. Other
applications include screening animals used in breeding programmes
as well as working dogs requiring good binaural hearing. ...
The information concerning normal hearing sensitivity has already
proved useful with additional dogs. Four CKCSs whose owners were
concerned about their dogs’ hearing have been tested subsequent to
this study. These dogs varied in age from four to eight years.
Hearing levels in the better ear were 55 dB nHL in three dogs and 80
dB nHL in the fourth dog. (Fig. 3. A dog set up for
auditory brainstem response hearing.)
Hearing Assessment in Cavaliers. Podell, M. CKCSC,USA Bulletin, Fall 1998; p. 21. Quote: "Deafness is an increasingly recognized problem of a number of pure-bred dogs, and more recently, has been documented as a congenital problem in the Cavalier King Charles Spaniel. Hearing loss may be due to a problem conducting sound waves through the middle ear (conductive loss) or to a problem related to the hearing receptors or nerve in the inner ear (sensorineural hearing loss). Conductive hearing loss usually is a problem related to ear infection(s) or aging. Sensorineural hearing loss occurs most commonly as a congenital disease; that is, the condition is present from birth. The loss can be complete, leading to deafness, or partial, leading to abnormal hearing function. ... Recently, a new finding has emerged in studying congenital deafness in Cavaliers. Rather than present with complete hearing loss in one or both ears, a particular line of dogs has developed a progressive hearing loss as they matured over first few years of life. These dogs were documented to have a normal BAER test as young puppies, using standard testing. However, over time, the dogs developed significant hearing loss, even to the point of being deaf. This type of hearing loss may be quite different from that of other breeds, in that the hearing nerve may be degenerating, rather than failure of formation or early degeneration of the inner ear receptors. This type of hearing loss may be similar to that seen in progressive nerve degeneration in young children and adolescents. This condition presents a unique problem in that puppies may test completely normal, and then be found to develop later onset hearing nerve degeneration after already passing on a potentially inherited disorder."
Congenital Deafness and Its Recognition. Strain, G.M. Veterinary Clinics of N.A.: Small Animal Practice - Spec. Iss., Pediatrics - July, 1999;29(4):895-907. Quote: "Sensory function in neonatal dogs and cats is primarily tactile, olfactory, and gustatory. The visual and auditory senses, although partially functional at birth, exhibit significant postnatal development: in the dog, the eyes do not open until a puppy is 8 to 10 days of age, the ear canals do not open until it is 12 to 13 days of age, and mature system function up through the cortex is not present until it is 3 months of age or 0Ider.18,22 Similar delays are seen in the cat. As a result, disorders of these systems frequently escape early detection. Deafness can be described as (1) congenital or late onset, (2) hereditary or acquired, and (3) conductive or sensorineural,14, 15 The most commonly occurring deafness forms in dogs and cats are congenital hereditary sensorineural deafness, late-onset acquired sensorineural deafness, and late-onset acquired conductive deafness. Distinguishing between hereditary and acquired deafness is generally not possible without breeding trials, although an assumption of hereditary deafness can be made in breeds with a high prevalence of deafness. The most common form of deafness in young dogs and cats is congenital hereditary sensorineural deafness, with acquired conductive and acquired sensorineural deafness appearing on rare occasions." (Includes CKCSs).
Advances in assessing canine degenerative hearing loss. M. Podell. Proc Am Coll Vet Intern Med. 1999; 17:291-293. Abiotrophic inherited congenital sensorineural deafness directly affects the hair cells of the spiral organ. The endocochlear potential is normal in these animals. The lesion is usually bilateral and occasionally also affects the hair cells of the vestibular system receptors. The inheritance usually involves an autosomal recessive gene. Although most of these abiotrophies cause deafness at a few weeks of age, occasionally the onset of deafnes occurs later in life. In the Cavalier King Charles Spaniel, deafness may not be apparent until 3 or 4 years of age.
Pet Care in the New Century: Cutting-Edge Medicine for Dogs and Cats. Shojai, AD. 2001, New Amer Library, p.185. Quote: "Dr. Michael Podell ... reports that a particular line of CKCSs seems to present a new type of deafness. Instead of the more typical complete hearing loss in one or both ears from birth, puppies have normal hearing at birth and develop a progressive hearing loss over the first few years of life, even to the point of becoming deaf. Dr. Podell suspects this type of hearing loss is caused by degeneration of the hearing nerve. The new condition poses a problem in screening such dogs to keep potential breeders from passing on the tendency to their offspring. Further studies are underway to identify tests that may be useful in predicting which dogs may develop such a hearing problem."
Deafness prevalence and pigmentation and gender associations in dog breeds at risk. George M. Strain. Vet. J. 2004; 167:23–32. (Includes CKCSs).
Review of the Brainstem Auditory Evoked Potential in the Dog. Wayne J. Wilson, Paul C. Mills. Amer. J. Vet. Resear. December 2005;66(12):2177-2187. Quote: There is growing interest in the use of the brainstem auditory evoked potential (BAEP) as a measure of auditory function in the dog. This interest is fuelled by the fact that the BAEP is: relatively easy to record, noninvasive, safe, mobile, brief in test time, objective, reliable, a sensitive and comprehensive index of neurological status, anatomically specific, cost effective (compared to some other objective measures of auditory function), generally independent of level of consciousness, resistant to drugs, and has the potential to be automated. Despite these advantages, the potential for error in the hands of inexperienced or inadequately trained clinicians remains significant. With this in mind, this paper aims to contribute to the clinician’s knowledge of the BAEP as it is applied to the dog, by reviewing BAEP theory, practice, current application, and future potential.
Veterinary Neuroanatomy and Clinical Neurology. Alexander de Lahunta, Eric N. Glass. Saunders 3d edit. July 2008; 438. Quote: "Congenital abiotrophic sensorineural deafness directly affects the hair cells in the spiral organ. ... The lesion is usually bilateral and occasionally also affects the hair cells of the vestibular system receptors. The inheritance usually involves an autosomal recessive gene. Although most of these abiotrophies cause deafness at a few weeks of age, occasionally the onset of deafness occurs later in life. In the cavalier King Charles spaniel, deafness may not be apparent until 3 or 4 years of age."
A Practical Guide to Canine and Feline Neurology. Curtis W. Dewey. John Wiley & Sons; 2008; 4-6. "Breed-associated neurologic abnormalities of dogs and cats. ... Cavalier King Charles Spaniels ... Congenital deafness."
Canine Inherited Disorders Database: http://www.upei.ca/~cidd/Diseases/nervous system disorders/deafness.htm
Post-anesthesia deafness in dogs and cats following dental and ear cleaning procedures. Cathryn K. Stevens-Sparks, George M. Strain. Vet. Anaesthesia & Analgesia. July 2010;37(4):347-351. Quote: "Objective: The present study was performed to document hearing loss in dogs and cats following procedures performed under anesthesia. Most cases of reported hearing loss were subsequent to dental and ear cleaning procedures. Study design: Prospective and retrospective case survey. Animals: Subjects were dogs and cats with deafness, personally communicated to one author, cases discussed on a veterinary information web site, and cases communicated through a survey of general practice and dental specialist veterinarians. Methods: Reported deafness cases were characterized by species (dog, cat), breed, gender, age, and dog breed size. Results: Sixty-two cases of hearing loss following anesthesia were reported between the years 2002 and 2009. Five additional cases were reported by survey respondents. Forty-three cases occurred following dental procedures. Sixteen cases occurred following ear cleaning. No relationship was observed between deafness and dog or cat breed, gender, anesthetic drug used, or dog size. Geriatric animals appeared more susceptible to post-anesthetic, post-procedural hearing loss. Conclusions: Deafness may occur in dogs and cats following anesthesia for dental and ear cleaning procedures, but the prevalence is low. The hearing loss appears to be permanent."
Deafness in Dogs & Cats. Strain, G.M. Nov. 2011. (Includes CKCSs).
Canine Deafness. George M. Strain. Vety.Clinics.Sm.Anim.Pract. Oct.2012. Quote: Conductive deafness, caused by outer or middle ear obstruction, may be corrected, whereas sensorineural deafness cannot. Most deafness in dogs is congenital sensorineural hereditary deafness, associated with the genes for white pigment: piebald or merle. The genetic cause has not yet been identified. Dogs with blue eyes have a greater likelihood of deafness than brown-eyed dogs. Other common forms of sensorineural deafness include presbycusis, ototoxicity, noise-induced hearing loss, otitis interna, and anesthesia. Definitive diagnosis of deafness requires brainstem auditory evoked response testing. ...
Electrodiagnostic Evaluation of Auditory Function in the Dog. Peter M. Scheifele, John Greer Clark. Vet.Clinics of N.A.:Sm.Anim.Prac. Nov. 2012; 42(6):1241-1257. Quote: "Given the high incidence of deafness within several breeds of dogs, accurate hearing screening and assessment is essential. In addition to brainstem auditory evoked response (BAER) testing, 2 other electrophysiologic tests are now being examined as audiologic tools for use in veterinary medicine: otoacoustic emissions and the auditory steady state response (ASSR). To improve BAER testing of animals and ensure an accurate interpretation of test findings from one test site to another, the establishment of and adherence to clear protocols is essential. The ASSR holds promise as an objective test for rapid testing of multiple frequencies in both ears simultaneously."
Canine Brainstem Auditory Evoked Responses are not Clinically Impacted by Head size or Breed. Debra L. Kemper, Peter M. Scheifele, John Greer Clark. Physiology & Behavior. Feb. 2013. 110-111:190-197. Quote: "Accurate assessment of canine hearing is essential to decrease the incidence of hereditary deafness in predisposed breeds and to substantiate hearing acuity. The Brainstem Auditory Evoked Response (BAER) is a widely accepted, objective test used in humans and animals for estimation of hearing thresholds and deafness diagnosis. In contrast to humans, testing and recording parameters for determination of normal values for canine hearing are not available. Conflicting information concerning breed and head size effects on canine BAER tests are major contributors preventing this normalization. The present study utilized standard head measurement techniques coupled with BAER testing and recording parameters modeled from humans to examine the effect canine head size and breed have on BAER results. Forty-three adult dogs from fourteen different breeds [including the cavalier King Charles spaniel] had head size measurements and BAER tests performed. The mean latencies compared by breed for waves I, II, III, IV, and V were as follows: 1.46±0.49msec, 2.52±0.54msec, 3.45±0.41msec, 4.53±0.83msec and 5.53±0.43msec, respectively. The mean wave I-V latency interval for all breeds was 3.69msec. All dogs showed similar waveform morphology, structures, including the presence of five waves occurring within 11 milliseconds after stimulus presentation and a significant trough occurring after Wave V. All of the waveform morphology for our subjects occurred with consistent interpeak latencies as shown by statistical testing. All animals had diagnostic results within the expected ranges for each wave latency and interwave interval allowing diagnostic evaluation. Our results establish that neither differences in head size nor breed impact determination of canine BAER waveform morphology, latency, or hearing sensitivity for diagnostic purposes. The differences in canine head size do not have a relevant impact on canine BAERs and are not clinically pertinent to management or diagnostic decisions."
The Effects of Magnetic Resonance Imaging Noise on Cochlear Function in Dogs. Chapter 2 of "Effects of acute and chronic noise exposure on cochlear function and hearing in dogs." Rebecca Elisabeth Venn. MSc(R) thesis pp 21-42, University of Glasgow. 2013. Quote: "In specialised veterinary hospitals, Magnetic Resonance Imaging (MRI) scanners are used daily in diagnostics of dogs. MRI scanners omit high levels of acoustic noise, which is known to be damaging to the hearing of human patients without effective ear protection. However, the effects of the MRI noise levels on the cochlear function and hearing of dogs is often overlooked and in many clinics, dogs are not provided with ear protection for the duration of their scan. The aim of this study was to assess the effects of MRI acoustic noise on the cochlear function of dogs, by Distortion Product Otoacoustic Emissions (DPOAE) testing dogs immediately before and after they underwent an MRI scan. ... Thirty-six dogs were included in the MRI group (mean age 3.9 years, median age 3 years, range 6 months to 10 years; mean bodyweight 16.9 kg, median bodyweight 13.8 kg, range 3.5 kg to 40.8 kg) and 17 dogs were included in the control group (mean age 6.2 years, median age 7 years, range 1 year to 12 years; mean bodyweight 25.1 kg, median bodyweight 23.6 kg, range 6.7 kg to 57.8 kg). There were 16 male dogs in the MRI group (44.4%) and 10 male dogs in the control group (58.8%). A variety of dog breeds were represented, with those represented more than once including three toy poodles, three Lhasa Apso dogs, four Labrador retrievers, two cocker spaniels, four Cavalier King Charles spaniels, two boxers and three cross-breeds in the MRI group, and two Labrador retrievers and two cross-breeds in the control group.... A group of control dogs undergoing a quiet procedure (but treated with the same range of anaesthetic drugs) were also tested. Post-MRI, the mean DPOAE of the dogs was reduced at all frequencies tested, significantly so at five (out of fourteen) frequencies, reflecting a reduction in cochlear function. Furthermore, at all frequencies tested, more than half of the ears exposed to MRI noise demonstrated a decrease in DPOAE. ... The results from this study indicate that exposure to noise during MRI in dogs results in a reduction in cochlear function, which is significant at multiple sound frequencies. ... Without repeat DPOAE testing of the dogs some weeks after their MRI, it is unknown whether this effect is temporary and reversible, or permanent. ... Evidence from human MRI noise exposure would suggest that this effect is temporary. The frequency region affected is likely influenced by the frequency of the noise spectra of the MRI. The demonstration that MRI noise results in some degree of hearing loss, albeit only assessed in the immediate post-MRI period in the present study, would suggest that all dogs having MRI studies performed should have ear protection as a standard precautionary measure."
The Canine Jaw-Ear Connection: The Malleomandibular and Tympanomandibular Ligaments. Cathryn Stevens-Sparks, George M. Strain. Anat. Rec. May 2014;297(5):876-891. Quote: "In the human, two ligaments derived from the first embryonic pharyngeal (branchial) arch that unite the mandible and temporomandibular joint (TMJ) with the middle ear have been identified as the discomalleolar ligament (DML) and sphenomandibular ligament (SML), also known as the malleomandibular ligament (MML), anterior ligament of the malleus (AML), and tympanomandibular ligament (TML). Neither of these structures has been previously described in the dog. The homologue of the human sphenomandibular ligament (SML) exists in the dog and is represented as a fibrous remnant of Meckel's cartilage. In the newborn puppy, the ligament is a true malleomandibular ligament (MML), extending from the medial mandible to the rostral process of the malleus with no intermittent attachments. In the adult dog, the ligament is entrapped within a bony passageway, likely due to the development and ossification of the tympanic bulla, making it difficult to grossly view the complete course of the ligament. The majority of the ligamentous fibers attach near the tympanic bulla in the adult dog, thus this portion of the ligament has been named the tympanomandibular ligament (TML). Those fibers of the ligament not attaching near the tympanic bulla appear to continue through a canal, located between the tympanic annulus and the surrounding tympanic bone, to become continuous with a connective tissue sheet within the cavity of the middle ear that has attachments to the malleus and incus. Tension on the adult canine TML did not result in movement of the malleus."
The effect of magnetic resonance imaging noise on cochlear function in dogs. R.E. Venn, A.R. McBrearty, D. McKeegan, J. Penderis. Vet.J. July 2014. Quote: "Noise produced by magnetic resonance imaging (MRI) scanners (which can peak at a sound pressure level of 131 dB) has been shown to cause noise-induced cochlear dysfunction in people. The aim of this study was to investigate whether noise produced during MRI had a deleterious effect on cochlear function in dogs, using distortion product otoacoustic emission (DPOAE) testing, which allows frequency specific, non-invasive assessment of cochlear function. A control group comprised dogs undergoing anaesthesia of a similar duration for quiet procedures. Thirty-six dogs (66 ears) and 17 dogs (28 ears) were included in the MRI and control groups respectively. There was a reduction in DPOAE at all frequencies tested in the MRI group; a similar effect was not evident in the control group. This reduction in the MRI group was statistically significant in five of the 14 frequencies assessed (P<0.05). Exposure to noise during MRI in dogs results in a significant reduction in cochlear function, although it is not known whether this is reversible or permanent. The frequency region affected is probably influenced by the frequency of the noise spectra of the MRI. The finding that MRI noise results in hearing loss, albeit only assessed in the immediate post-MRI period, would suggest that all dogs undergoing MRI studies should have ear protection as a standard precaution. What is not evident from the present study is the longer-term effect of excessive MRI noise on hearing and further studies are also required to assess the efficacy of ear protection to protect against the effects of MRI noise in dogs." (See also the 2013 Rebecca Elisabeth Venn master's thesis above.)
The genetics of deafness in domestic animals. George M. Strain. Frontiers in Vet. Sci. September 2015. Quote: "Although deafness can be acquired throughout an animal’s life from a variety of causes, hereditary deafness, especially congenital hereditary deafness, is a significant problem in several species. Extensive reviews exist of the genetics of deafness in humans and mice, but not for deafness in domestic animals. Hereditary deafness in many species and breeds is associated with loci for white pigmentation, where the cochlear pathology is cochleo-saccular. In other cases, there is no pigmentation association and the cochlear pathology is neuroepithelial. Late onset hereditary deafness has recently been identified in dogs and may be present but not yet recognized in other species. Few genes responsible for deafness have been identified in animals, but progress has been made for identifying genes responsible for the associated pigmentation phenotypes. Across species, the genes identified with deafness or white pigmentation patterns include MITF, PMEL, KIT, EDNRB, CDH23, TYR, and TRPM1 in dog, cat, horse, cow, pig, sheep, ferret, mink, camelid, and rabbit. Multiple causative genes are present in some species. Significant work remains in many cases to identify specific chromosomal deafness genes so that DNA testing can be used to identify carriers of the mutated genes and thereby reduce deafness prevalence. ... Cavalier King Charles spaniels: A form of late onset conductive hearing loss known as primary secretory otitis media (PSOM) appears in Cavalier King Charles spaniels, although at least one case each has also been reported in a Dachshund, a boxer, and a Shih Tzû. No proven hereditary cause has been identified, but the preponderance of occurrence in the one breed supports this as a possible factor. Several single-gene otitis media-causing mouse mutants have been identified and there is a suggestion of a hereditary component in OM in humans. A correlation between PSOM and pharyngeal conformation has been suggested, possibly from a thick soft palate and reduced nasopharyngeal aperture, which might argue against a hereditary basis. Because the phenotype of the Cavalier includes white pigment that probably results from a recessive allele of the S locus, affected dogs may incorrectly be thought to exhibit the congenital CS pathology. Primary secretory otitis media, also known as glue ear, results from the development of a solid viscous plug in the middle ear due to either increased production of mucus by the middle ear lining or reduced drainage through the auditory (Eustachian) tube. Signs include hearing loss, neck scratching, otic pruritus, head shaking, abnormal yawning, head tilt, facial paralysis, or vestibular disturbances. Testing has confirmed that the hearing loss is conductive. Implantation of tympanostomy tubes has been successful in a small number of cases, but treatment is typically by myringotomy followed by flushing of the mucus plug, repeated as needed. No studies have been reported of genetic association studies."
Clonal Expansion of Lgr5-Positive Cells from Mammalian Cochlea and High-Purity Generation of Sensory Hair Cells. Cell Reports. February 2017;18(8):1917-1929. Quote: Death of cochlear hair cells, which do not regenerate, is a cause of hearing loss in a high percentage of the population. Currently, no approach exists to obtain large numbers of cochlear hair cells. Here, using a small-molecule approach, we show significant expansion (>2,000-fold) of cochlear supporting cells expressing and maintaining Lgr5, an epithelial stem cell marker, in response to stimulation of Wnt signaling by a GSK3β inhibitor and transcriptional activation by a histone deacetylase inhibitor. The Lgr5-expressing cells differentiate into hair cells in high yield. From a single mouse cochlea, we obtained over 11,500 hair cells, compared to less than 200 in the absence of induction. The newly generated hair cells have bundles and molecular machinery for transduction, synapse formation, and specialized hair cell activity. Targeting supporting cells capable of proliferation and cochlear hair cell replacement could lead to the discovery of hearing loss treatments.
Normative brainstem auditory evoked responses in Cavalier King Charles Spaniels with Chiari-like malformation. Lynette K. Cole; Susan Wagner; Sarah Moore; Ronaldo da Costa; Eric Hostnik; Laura Selmic. 2022 ACVIM Forum Research Report Program. J. Vet. Intern. Med. October 2022; doi: 10.1111/jvim.16538. Quote: Background: Hearing in dogs can be evaluated using brainstem auditory evoked response (BAER) testing. Cavalier King Charles spaniels (CKCS) are prone to Chiari-like malformation (CM). Hypothesis/Objectives: To evaluate hearing loss in CKCS, BAER parameters are needed in reference to presence or absence of CM. The purpose was to establish normative BAER data. The specific aim was to determine if BAER indices differed based on CM grade. We hypothesized that CM would result in latency differences in CKCS with CM grade 2 (CM2) compared to those with CM0 and CM1. Animals: Twenty CKCS without apparent hearing abnormalities assessed by owner. METHODS: Prospective case series. Under general anesthesia, CKCS underwent CT scan (assess middle ear), BAER testing, MRI (assess presence/grade of CM). Results: Nine (45%) CKCS had CM1; 11 (55%) had CM2. All had at least one morphologic abnormality in waveforms. Absolute and interpeak latencies were reported for all CKCS and compared between CM grades. Median threshold for CKCS with CM1 was 39 and CM2 was 46. Absolute latencies for CKCS with CM2 were consistently longer than those for CKCS with CM1 with exception of Wave II, V at 33 dB. Significant differences were found for wave V at 102 dB, wave II at 74 dB. Interpeak latency comparisons were inconsistent between CM1 and CM2. Conclusions & Clinical Importance: Normative BAER data for CKCS with CM1 and CM2 were established. Results suggest CM impacts BAER latency results, but influence is not always statistically significant or predictable.
Chiari-like malformation in Cavalier King Charles Spaniels impacts brainstem auditory-evoked response latency results. Lynette K. Cole, Susan O. Wagner, Sarah A. Moore, Ronaldo DaCosta, Eric T. Hostnik, Laura E. Selmic. Am. J. Vet. Res. May 2023; doi: 10.2460/ajvr.23.03.0064. Quote: Objective: To evaluate hearing loss in Cavalier King Charles Spaniels (CKCS), breed-specific brainstem auditory-evoked response (BAER) testing parameters are needed to help assess the Chiari-like malformation (CM) grade. The purpose of this study was to establish breed-specific BAER data and to determine if BAER indexes differed based on the CM grade. We hypothesized that there would be latency differences based on the CM grade. Animals: 20 CKCS without apparent hearing abnormalities as assessed by the owners. Procedures: Under general anesthesia, CKCS underwent a CT scan (to assess the middle ear), BAER testing, and MRI (to assess the grade of CM). Results: No CKCS had CM0. Nine (45%) CKCS had CM1; 11 (55%) had CM2. All had at least 1 morphologic abnormality in waveforms. Absolute and interpeak latencies were reported for all CKCS and compared between CM grades. The median threshold for CKCS with CM1 was 39 and for CM2 was 46. Absolute latencies for CKCS with CM2 were consistently longer than those for CKCS with CM1 with the exception of waves II and V at 33 dB. Significant differences were found for wave V at 102 dB ( P = .04) and wave II at 74 dB (P = .008). Interpeak latency comparisons were inconsistent between CM1 and CM2. Clinical Relevance: Breed-specific BAER data for CKCS with CM1 and CM2 were established. The results suggest that CM impacts BAER latency results, but the influence of the malformation is not always statistically significant or predictable. ... Our study established breed-specific data of BAER measures for CKCS with CM1 and CM2. Specifically, we now have BAER data of absolute and interpeak latencies, as well as the threshold in CKCS based on CM grade. In addition, wave V latency intensity function was constructed with respect to CM grade that can be utilized to assess the presence and type of hearing loss in the CKCS. ... In conclusion, BAER data in CKCS with respect to CM grade have been established. BAER indexes differed based on CM grade in CKCS, but the influence of the grade of the malformation, be it CM1 or CM2, is not always statistically significant or predictable.


CONNECT WITH US